Note: In this section, we will be talking about the elements
present in the reactivity series that are only in the IGCSE syllabus.
A major problem all chemistry students face in the
topic “The Reactivity series” is memorizing the series itself. Not many
are fortunate enough to have the capability of learning the whole series by
heart and keeping it in mind for the long term. The good news is, there are
ways you can learn the reactivity series and never forget it again.
The
Reactivity Series
To make things clear, let us start with the topic
itself. The reactivity series is a list of elements (mainly metals) in an order
of decreasing reactivity.
|
As the elements go from bottom to top:
- They increase in reactivity
- They lose electrons more readily to form positive ions
- They corrode more readily
- They require more energy and different separate methods to be separated from their ores
- They become stronger reducing agents (electron donors)
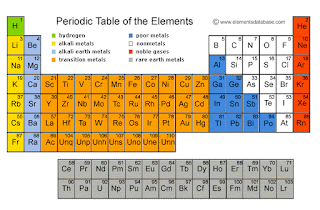
The Periodic Table
You might wonder what role the periodic table plays in
learning the Reactivity series. Well it plays a big one!
Everyone has their own ways of learning about a
certain topic. However, in the end of the day we all use the same technique. A
pattern. It is always easier to learn something when you see a pattern to it
and that is exactly what we are going to use the periodic table for.
Have a look at these two and try to compare the
elements in the Reactivity series with their respective positions in the
periodic table.
You will see that the first
three elements- K, Na and Li – are present in the same group, that is group 1.
In the second group, we have the two elements- Ca and Mg. At this point you
will realize that you have already learned the first five elements in the Reactivity series, they were neighbors the whole time!
Note: We will be skipping the two
elements, Carbon and Hydrogen temporarily for our own convenience.
After we are done with
Magnesium, we will be jumping to Aluminium over the transition metals, which is
present in group 3. At the bottom left of Aluminium, we have Zinc. The pattern
we are trying to achieve from this point onward might seem fuzzy, but it is
very simple. From Zinc, we go left towards Iron and then again back right to
copper. From here, we just go downward-Cu, Ag, Au.
Adding carbon and hydrogen
is just as simple. The first four elements ends with the letter ‘m’. So just
add the Carbon after the last ‘m’, which is Aluminium. Hydrogen on the other
hand can be remembered easily when you place it on top of Copper.
Mnemonics
Mnemonics is another popular
and fun way of interacting with obstacles like the reactivity series. It is
used as a tool to help memorize a list by remembering specific letters (usually
the first) to make up another sentence. For example:
My Very Eager Mother Jumped (into the) SUN Neptune
Mercury Earth Jupiter Saturn
Venus Mars Uranus
Similarly, if we apply the
same technique with the reactivity series, we can get something like this:
People
|
Potassium
|
Say
|
Sulfur
|
Little
|
Lithium
|
Children
|
Calcium
|
Make
|
Magnesium
|
A
|
Aluminium
|
Carbon
|
|
Zebra
|
Zinc
|
I’ll
|
Iron
|
Hydrogen
|
|
Constantly
|
Copper
|
Sniffing
|
Silver
|
Giraffes
|
Gold
|
The above table shows only
an example, you will find more mnemonics like this in the internet.
Hope this helps. Study hard
and good luck with your exams.


No comments:
Post a Comment